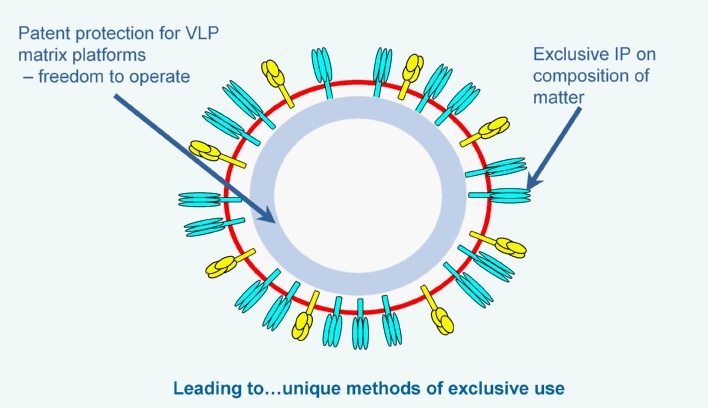
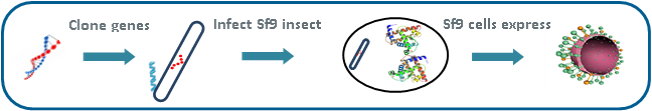
Another example of the power of our Sf9/BV platform is our ability to produce influenza virus-like particles (VLPs) using similar techniques. However, instead of producing single-target protein nanoparticles, as with our Rabies vaccine, scientists incorporated the gene sequences of multiple immunogenic proteins, together with universal matrix (M1) assembly proteins, to produce more complex envelope particle vaccines. Our influenza VLP vaccines incorporate the genetic sequences of the strain specific hemagglutinin (HA) and neuraminidase (NA) surface proteins. Traditional egg-based vaccines contain meaningful levels of HA protein, but little to no NA or M1, which may make them less immunogenic and effective. The HA sequence in our VLPs is the same as in the wild-type virus and could prove to be more effective or immunogenic than influenza vaccines produced using egg or mammalian cell-lines, which alter HA. In addition, the NA and M1 in our VLPs may play a role in reducing the severity of the disease by inducing antibody responses and cell mediated immunity. NA and M1 are both highly conserved, and immunity to these viral components may help provide additional protection throughout an entire influenza season, even as strains mutate.
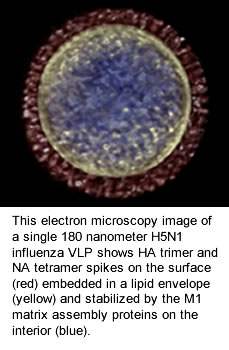
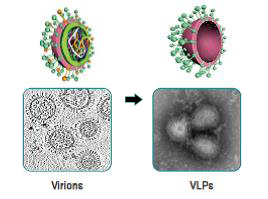
Once expressed, the HA, NA and M1 proteins self-assemble and bud off the insect cell. The end result is highly immunogenic spherical structures that are roughly the same size as influenza viruses, with a lipid bilayer membrane expressing the HA and NA proteins on the surface, stabilized by the matrix assembly protein. Because there is no genetic material (DNA or RNA) in these VLPs, it is impossible for them to infect host cells and cause disease. Instead, their virus-like qualities activate the immune system to stimulate both antibody B-cell and cellular T-cell responses. Finally, because of the structure and components of our VLPs, they may have greater immunogenicity in two vulnerable populations – pediatrics and older adults.The insect cell/BV recombinant platform is well-established in the biopharmaceutical industry and is the basis for several licensed biologic products and vaccines, including a vaccine licensed for Human Papilloma Virus (HPV), which showed >95% effectiveness in reducing the incidence of cervical cancer. We can use its insect cell/BV technology to produce a wide range of vaccine candidates derived from viruses, bacteria and protozoan parasites.
Recombinant VLPs lack Influenza virus genome for replication